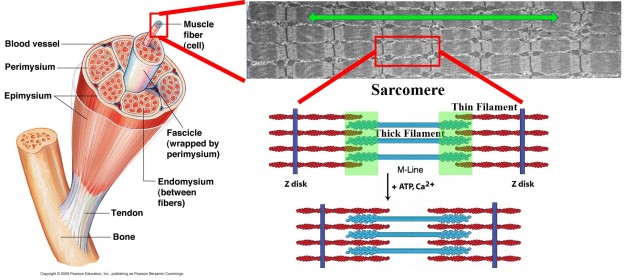
Whenever we go to the gym, pick up our pug, or hit the snooze button for the 10th time, our muscles produce forces against our bones, which lead to movement.
We still are not exactly sure how this all works. I study the function of a muscle protein called titin. We believe that this protein can answer a lot about how muscle force is produced, transmitted and regulated. See this review paper for all the details.
I use many different approaches to study muscles.
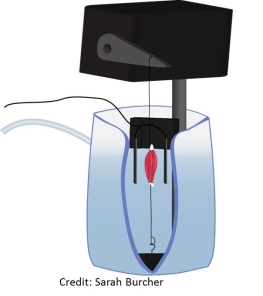
To study titin’s role in force production, I study mice with a small deletion in titin. These muscles are small, and we tie them to an apparatus that can activate them, change their length, and measure force. This basic setup is the foundation for many muscle-level experiments.
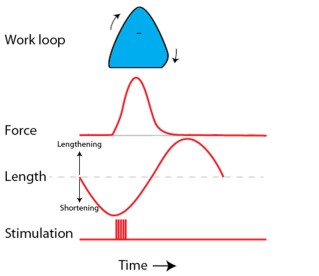
For one project, I assessed titin’s role during muscle braking (as done when walking downhill). I did this using the work loop method and produced negative work during stretch-shortening cycles. What happened? When we modified titin, it really decreased properties associated with muscle braking. See the results and assess for yourself.
I also evaluated titin’s role for force production and force transmission. The idea is a taboo thought for some scientists! For these studies I used whole muscles and fibers (cell). The results of these studies will be published soon.
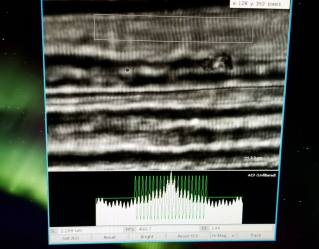
Sometimes, it is best to just go to the source: using low angle Xray diffraction, we can shoot Xrays through muscle fibers and interpret the scatter of the beam to understand what the motor components are doing during muscle activation! We traveled to the BiCAT lab at the Advanced Photon Source, Argonne National Labs (the USA particle accelerator) to do this research. This study is ongoing, but I will say that the preliminary results are FASCINATING.
